Orexigenic effect via interaction with nuclei within the hypothalamus1





The active ingredient in Mirataz® is mirtazapine
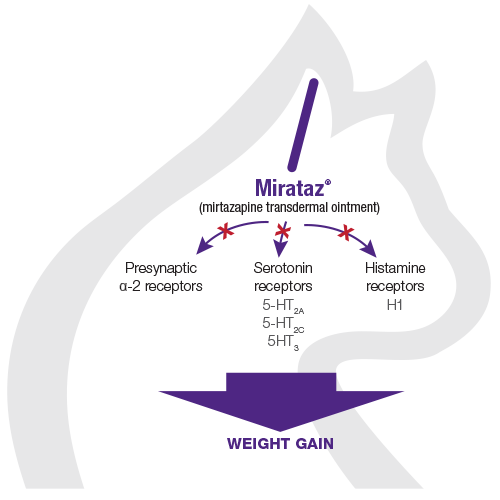
The pharmacodynamic action of mirtazapine involves antagonism of several receptor sites
Antagonism of presynaptic α-2 receptors, serotonin receptors (5-HT2A, 5-HT2C, 5-HT3), and histamine receptors (H1) by mirtazapine has been demonstrated to result in:
Enhanced release of both serotonin (5-HT) and noradrenaline (NE)2
Watch this video to learn how the pharmacodynamic action of mirtazapine helps lead to feline weight gain.
While not FDA approved, human and compounded versions of mirtazapine have been used off-label but may not be ideal for most cats
Human tablets must be split or broken, which may result in:
Inaccurate dosing, which has been shown to result in accidental overdose and toxicity3
Unknown user safety to humans handling cut or broken pills
Unknown drug distribution in pill fragments
Oral products placed on food will only work if the cat is eating and fed individually
Liquids by mouth may not be any easier than pilling
Compounded transdermal mirtazapine has been shown to result in inconsistent and highly variable blood mirtazapine levels4
Mirataz gives your clients an option for one less oral medication for their cats
Click here to read the “Dear Veterinarian” letter the FDA distributed regarding the benefits of using FDA-approved drugs – specifically using Mirataz versus compounded formulations of mirtazapine.
Mirataz is indicated for the management of weight loss in cats.
Important Safety Information
Mirataz® (mirtazapine transdermal ointment) is for topical use in cats only under veterinary supervision. Do not use in cats with a known hypersensitivity to mirtazapine or any of the excipients. Do not use in cats treated with monoamine oxidase inhibitors (MAOIs). Not for human use. Keep out of reach of children. Wear gloves when handling/applying, wash hands after and avoid contact between the treated cat and people or other animals for 2 hours following application. Use with caution in cats with hepatic and kidney disease. Cat’s food intake should be monitored upon discontinuation. Safety has not been evaluated in cats less than 2 kg, less than six months of age or in breeding, pregnant or lactating cats. The most common adverse reactions observed during clinical trials were application site reactions, behavioral abnormalities (vocalization and hyperactivity) and vomiting. For product label, including complete safety information, click here.
References
1. Agnew W, Korman R. Pharmacological appetite stimulation: rational choices in the inappetant cat. J Feline Med Surg. 2014;16(9):749–756.
2. de Boer T. The pharmacologic profile of mirtazapine. J Clin Psychiatry. 1996;57(suppl 4):19-25.
3. Ferguson LE, McLean MK, Bates JA, et al. Mirtazapine toxicity in cats: retrospective study of 84 cases (2006-2011). J Feline Med Surg. 2016;18(11):868-874.
4. Benson KK, Zajic LB, Morgan PK, et al. Drug exposure and clinical effect of transdermal mirtazapine in healthy young cats: a pilot study. J Feline Med Surg. 2017;19(10):998-1006.

US Patent 10,603,272
Mirataz® is a registered trademark of Dechra Ltd.
Interesting links
Here are some interesting links for you! Enjoy your stay :)Pages
- A little weight loss can be a big deal
- About Feline Weight Loss
- Click to learn how easy it is to apply Mirataz.
- Dosing and Administration
- Efficacy
- Fan & Follower Policy
- For Cat Owners – Video
- Frequently Asked Questions
- Home
- Learn how to identify changes in your cat’s habits.
- Mechanism of Action
- Mirataz News
- Mirataz PI
- Mirataz Thank You
- Mirataz Thank You Digital Tool Kit
- Mirataz® (mirtazapine transdermal ointment)
- Resources
- Safety
KindredBio proudly supports
Rabies Free Africa

